Please wait a moment until all data is loaded. This message will disappear when all data is loaded.
Please wait a moment until the data is sorted. This message will disappear when the data is sorted.
2 genotypes ICPL 84023 and ICP 301 tolerant to waterlogging stress, and 2 genotypes ICP 7035 and Pusa 207 susceptible to waterlogging stress. Pattern of variation in reducing sugar content in the 4 genotypes is parallel to sucrose synthase activity. ICPL 84023 and ICP 301 also show fewer declines in total and non-reducing sugars and greater increase in reducing sugar and SuSy activity than ICP 7035 and Pusa 207
-
cloning of sucrose synthase gene fragments. Sorghum sucrose synthase gene fragment I shares homology with other cereal sucrose synthase at the exon positions 6, 7, 8, 9 and 10. Sorghum sucrose synthase fragment II shares homology from exon 2 to 6
-
constitutive expression in Saccharomyces cerevisiae strain 22574dsus1
-
DNA and amino acid sequence determination and analysis, phylogenetic analysis
expressed in Escherichia coli BL21(DE3) and Saccharomyces. cerevisiae 22574d cells
-
expressed in Escherichia coli BL21(DE3) cells
expressed in Escherichia coli BL21-Gold (DE3) cells
expressed in Escherichia coli DH5alpha cells
-
expressed in Escherichia coli Tuner(DE3) cells
Thermosynechococcus vestitus
-
expressed in Nicotiana tabacum cultivar Havana 425
-
expressed in Oryza sativa cultivar Zhonghua11
-
expression in Agrobacterium tumefaciens
expression in Escherichia coli
gene CmSS1, DNA and amino acid sequence determination and analysis, RT-PCR, RACE, and real time PCR analysis
-
gene MtSucS1, expression analysis in wild-type and antisense plants
-
gene PsnSuSy2, DNA and amino acid sequence determination and analysis, phylogenetic analysis and tree, recombinant overexpression of poplar xylem sucrose synthase in Nicotiana tabacum via Agrobacterium tumefaciens-mediated transformation leads to a thickened cell wall and increased height of transgenic plants, phenotypic changes in PsnSusy2 transgenic lines. PsnSuSy2 expression levels and altered wood properties in stem segments from the different transgenic lines, real-time quantitative PCR analyses of tissues and transgenic lines, overview
-
gene ss2, phylogenetic analysis, recombinant expression of His-tagged enzyme in Escherichia coli strain BL21(DE3) using plasmid pNESS2
gene Sus, DNA and amino acid sequence determination and analysis, tissue-specific expression analysis
-
gene SUS1, expression analysis in various rice tissues using real-time quantitative RT-PCR
gene sus1, expression of wild-type enzyme and mutant S11D in Escherichia coli strain BL21(DE3) reveals monosaccharides D-ribulose, D-tagatose, L-glucose, and L-rhamnose as additional acceptor substrates, expression of wild-type SuSy1 and SuSy1 S11A mutant in Saccharomyces cerevisiae strain BY4741 with phosphorylation of the wild-type enzyme at Ser11
gene sus1, quantitative expression analysism, genotyping and phenotype-genotype relationship at different environmental conditions
gene SUS2, expression analysis in various rice tissues using real-time quantitative RT-PCR
gene sus2, quantitative expression analysism, genotyping and phenotype-genotype relationship at different environmental conditions
gene SUS3, DNA and amino acid sequence determination and analysis, sequence comparisons and phylogenetic tree of plant SUS enzymes, real-time quantitative PCR enzyme expression analysis, antisense expression of SUS3 in Cucumber sativus plants transformed via Agrobacterium tumefaciens strain LBA4404
gene SUS3, expression analysis in various rice tissues using real-time quantitative RT-PCR
gene SUS4, expression analysis in various rice tissues using real-time quantitative RT-PCR
gene sus4, quantitative expression analysism, genotyping and phenotype-genotype relationship at different environmental conditions
gene SUS4, recombinant ectopic expression of the enzyme in Zea mays seed endosperm. Transgenic developing seeds exhibit a significant increase in SuSy activity, the transgenic seeds accumulate 10-15% more starch at the mature stage and contain a higher amylose/amylopectin balance than wild-type maize seeds, while no significant changes are detected in the transgenic seeds in the content of soluble sugars, and in activities of starch metabolism-related enzymes when compared with wild-type seeds, overview
gene SUS5, expression analysis in various rice tissues using real-time quantitative RT-PCR
gene sus5, quantitative expression analysism, genotyping and phenotype-genotype relationship at different environmental conditions
gene SUS6, expression analysis in various rice tissues using real-time quantitative RT-PCR
gene sus6, quantitative expression analysism, genotyping and phenotype-genotype relationship at different environmental conditions
gene SuSy, quantitative real-time PCR in wild-type and transgenic plant leaves
-
gene SuSyAc, phylogenetic analysis, recombinant expression of C-terminally His6-tagged enzyme in Escherichia coli strain BL21(DE3)
gene SuSyDa, phylogenetic analysis, recombinant expression of C-terminally His6-tagged enzyme in Escherichia coli strain BL21(DE3)
gene SuSyMr, phylogenetic analysis, recombinant expression of C-terminally His6-tagged enzyme in Escherichia coli strain BL21(DE3)
gene SuSyNe, phylogenetic analysis, recombinant expression of C-terminally His6-tagged enzyme in Escherichia coli strain BL21(DE3)
genotyping of wild-type and thermotolerant lines
-
Nicotiana tabacum cv. xanthi.transgenic plants expressing either gene under the control of a tandem repeat cauliflower mosaic virus 35S promoter (2*35S) or a xylem-localized 4CL promoter (4-coumarate:CoA ligase) are generated. SuSy has the potential to increase overall plant growth and thus increase the total cellulose yield attainable from an individual plant
-
overexpression of SuSy in transgenic plants
-
overexpression of the Gossypium hirsutum SuSy gene under control of two promoters in hybrid poplar Populus alba x grandidentata leading to significantly increased SuSy enzyme activity in developing xylem and to increased secondary cell wall cellulose content
-
production of recombinant His6-SUS2 protein (rSUS2) in Escherichia coli
-
recombinant overexpression in leaves of Nicotiana tabacum cv. SR1 plants via transfection with Agrobacterium tumefaciens strain GV3013
sus3, recombinant expression of wild-type and mutant enzymes in Pichia pastoris
the gene is artificially synthesized for optimal recombinant expression in Escherichia coli strain Tuner (DE3)
Thermosynechococcus vestitus
-
-

-
DNA and amino acid sequence determination and analysis, phylogenetic analysis

-
DNA and amino acid sequence determination and analysis, phylogenetic analysis
-
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
DNA and amino acid sequence determination and analysis, phylogenetic analysis
expressed in Escherichia coli BL21(DE3) cells

-
expressed in Escherichia coli BL21(DE3) cells
-
expressed in Escherichia coli BL21(DE3) cells
-
expressed in Escherichia coli BL21(DE3) cells
-
expressed in Escherichia coli BL21(DE3) cells
-
expressed in Escherichia coli BL21(DE3) cells
expressed in Escherichia coli BL21(DE3) cells
-
expressed in Escherichia coli BL21(DE3) cells
-
expressed in Escherichia coli BL21(DE3) cells
expressed in Escherichia coli BL21-Gold (DE3) cells

expressed in Escherichia coli BL21-Gold (DE3) cells
expression in Escherichia coli

-
expression in Escherichia coli
Arachnis hookeriana x Ascocenda Madame Kenny
-
expression in Escherichia coli
expression in Escherichia coli


 results (
results (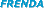 results (
results ( top
top





